Internal conversion
Internal conversion is a radioactive decay process where an excited nucleus interacts with an electron in one of the lower atomic orbitals, causing the electron to be emitted from the atom. Thus, in an internal conversion process, a high-energy electron is emitted from the radioactive atom, but without beta decay taking place. For this reason, the high-speed electrons from internal conversion are not beta particles (β particles), since the latter come from beta decay. Since no beta decay takes place in internal conversion, the element atomic number does not change, and thus (as is the case with gamma decay) no transmutation of one element to another is seen. Also, no neutrino is emitted in internal conversion.
Internally converted electrons do not have the characteristic energetically-spread spectrum of β particles, which results from varying amounts of decay-energy being carried off by the neutrino (or antineutrino) in beta decay. Internally converted electrons, which carry a fixed fraction of the characteristic decay energy, have a well-specified discrete energy. The energy spectrum of a β particle is thus a broad hump, extending to a maximum decay energy value, while the spectrum of internally converted electrons is a sharp peak.
Contents |
Mechanism
In the internal conversion process, the wavefunction of an inner shell electron penetrates the nucleus (i.e. there is a finite probability of the electron in an s atomic orbital being found in the nucleus) and when this is the case, the electron may couple to the excited state and take the energy of the nuclear transition directly, without an intermediate gamma ray being produced first.
As an electromagnetic quantum process, the process of imparting energy to the electron may be seen as taking place by means of a virtual photon, but in that sense the photon involved can be considered as a "virtual gamma ray", which never appears except as a feature of an equation, rather than a directly measurable particle. The kinetic energy of the emitted electron is equal to the transition energy in the nucleus, minus the binding energy of the electron.
Most internal conversion electrons come from the K shell (1s state, see electron shell), as these two electrons have the highest probability of being found inside the nucleus. After the electron has been emitted, the atom is left with a vacancy in one of the inner electron shells. This hole will be filled with an electron from one of the higher shells and subsequently a characteristic x-ray or Auger electron will be emitted.
When the process is expected
Internal conversion is favoured when the energy gap between nuclear levels is small, and is also the primary mode of de-excitation for 0+→0+ (i.e. E0) transitions (i.e., where excited nuclei are able to rid themselves of energy without changing electric and magnetic moments in certain ways) with insufficient energy to decay by pair production. It is the predominant mode of de-excitation whenever the initial and final spin states are the same, but the multi-polarity rules for nonzero initial and final spin states do not necessarily forbid the emission of a gamma ray in such a case.
The tendency towards internal conversion can be determined by the internal conversion coefficient, which is empirically determined by the ratio of de-excitations that go by the emission of electrons to those that go by gamma emission.
The internal conversion process competes with gamma decay. This competition is quantified in the form of the internal conversion coefficient which is defined as 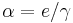 where
where 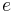 is the rate of conversion electrons and
is the rate of conversion electrons and 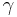 is the rate of gamma-ray emission observed from a decaying nucleus. For example, in the decay of an excited state of the nucleus of 125I, 7% of the decays emit energy as a gamma ray, while 93% release energy as conversion electrons. Therefore, this excited state of 125I has an internal conversion coefficient of
is the rate of gamma-ray emission observed from a decaying nucleus. For example, in the decay of an excited state of the nucleus of 125I, 7% of the decays emit energy as a gamma ray, while 93% release energy as conversion electrons. Therefore, this excited state of 125I has an internal conversion coefficient of 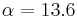 . Internal conversion coefficients are observed to increase for increasing atomic number (Z) and decreasing gamma-ray energy. As one example, IC coefficients are calculated explicitly for 55Fe, 67Ga, 99mTc, 111In, 113mIn, 115mIn, 123I, 125I, 193mPt, 201Tl and 203Pb by Howell (1992) using Monte Carlo methods (for 55Fe the IC coefficient is zero).
. Internal conversion coefficients are observed to increase for increasing atomic number (Z) and decreasing gamma-ray energy. As one example, IC coefficients are calculated explicitly for 55Fe, 67Ga, 99mTc, 111In, 113mIn, 115mIn, 123I, 125I, 193mPt, 201Tl and 203Pb by Howell (1992) using Monte Carlo methods (for 55Fe the IC coefficient is zero).
The energy of the emitted gamma-ray is regarded as a precise measure of the difference in energy between the excited states of the decaying nucleus. However, this is not true in the case of conversion electrons. The energy of a conversion electron is given as 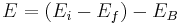 where
where 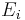 and
and 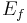 are the energies of the nucleus in its initial and final states, respectively, while
are the energies of the nucleus in its initial and final states, respectively, while 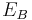 is the binding energy of the electron.
is the binding energy of the electron.
Similar processes
This internal conversion process is also not to be confused with the similar photoelectric effect, which also may occur with gamma radiation associated electron emission, in which an incident gamma photon emitted from a nucleus interacts with an electron, expelling the electron from the atom. Thus, gamma photoelectric effect electron emission may also cause high-speed electrons to be emitted from radioactive atoms without beta decay. However, in internal conversion, the nucleus does not first emit an intermediate real gamma ray, and therefore need not change angular momentum or electric moment.
Also, electrons from the gamma photoelectric effect show a spread in energy, depending on how much energy has been imparted to the ejected electron by the gamma ray which interacts with it—an amount which is variable depending on the angle of gamma photon scattering from the electron (see Compton scattering). Further, a gamma ray is still emitted in photoelectric processes, but one which possesses a fraction of the energy than the gamma ray which left the nucleus. By contrast, in internal conversion, as noted, no gamma ray is emitted at all and the electron energy is fixed at a single, typical value.
Auger electrons, which may also be produced after an internal conversion, arise from a mechanism that is different from that of internal conversion, but is analogous to it. Internal conversion electrons arise when an intense electric dipole field inside the nucleus accelerates an electron which has penetrated the nucleus, to remove it from the atom. Auger electrons similarly arise when an electric field is produced within an atom's electron cloud due to loss of another electron, and this field again induces the acceleration and removal of yet another of the atom's atomic orbital electrons. Like internal conversion electrons, Auger electrons also emerge in a sharp energy peak.
The electron capture (EC) process also involves an inner shell electron, which in this case is retained in the nucleus (changing the atomic number) and leaving the atom (not the nucleus) in an excited state. The atom can relax by X-ray emission and/or by Auger electron emission. Unstable nuclei can usually decay through both IC and EC processes.
See also
References
Krane, Kenneth S. (1988). Introductory Nuclear Physics. J. Wiley & Sons. ISBN 0-471-80553-X.
L'Annunziata, Michael F. et al. (2003). Handbook of Radioactivity Analysis. Academic Press. ISBN 0124366031.
R.W.Howell, Radiation spectra for Auger-electron emitting radionuclides: Report No. 2 of AAPM Nuclear Medicine Task Group No. 6, 1992, Medical Physics 19(6), 1371-1383
External links
|
||||||||||||||||